Syntegon launches new MLD Advanced for RTU syringes

Supplier News
Following the first virtual presentation at Interphex, Syntegon is now introducing its new filling machine for ready-to-use (RTU) nested syringes to the market.
The MLD Advanced meets the increasing requirements of pharmaceutical manufacturers for high output with 100 percent in-process control (IPC). “Especially with high-value medicines, it is essential that each drop is filled and weighed optimally,” explains Markus Burkert, Product Manager at Syntegon. “That's why we have combined the MLD platform, which was previously used primarily for cartridges and vials, with our technologies for syringe filling.”
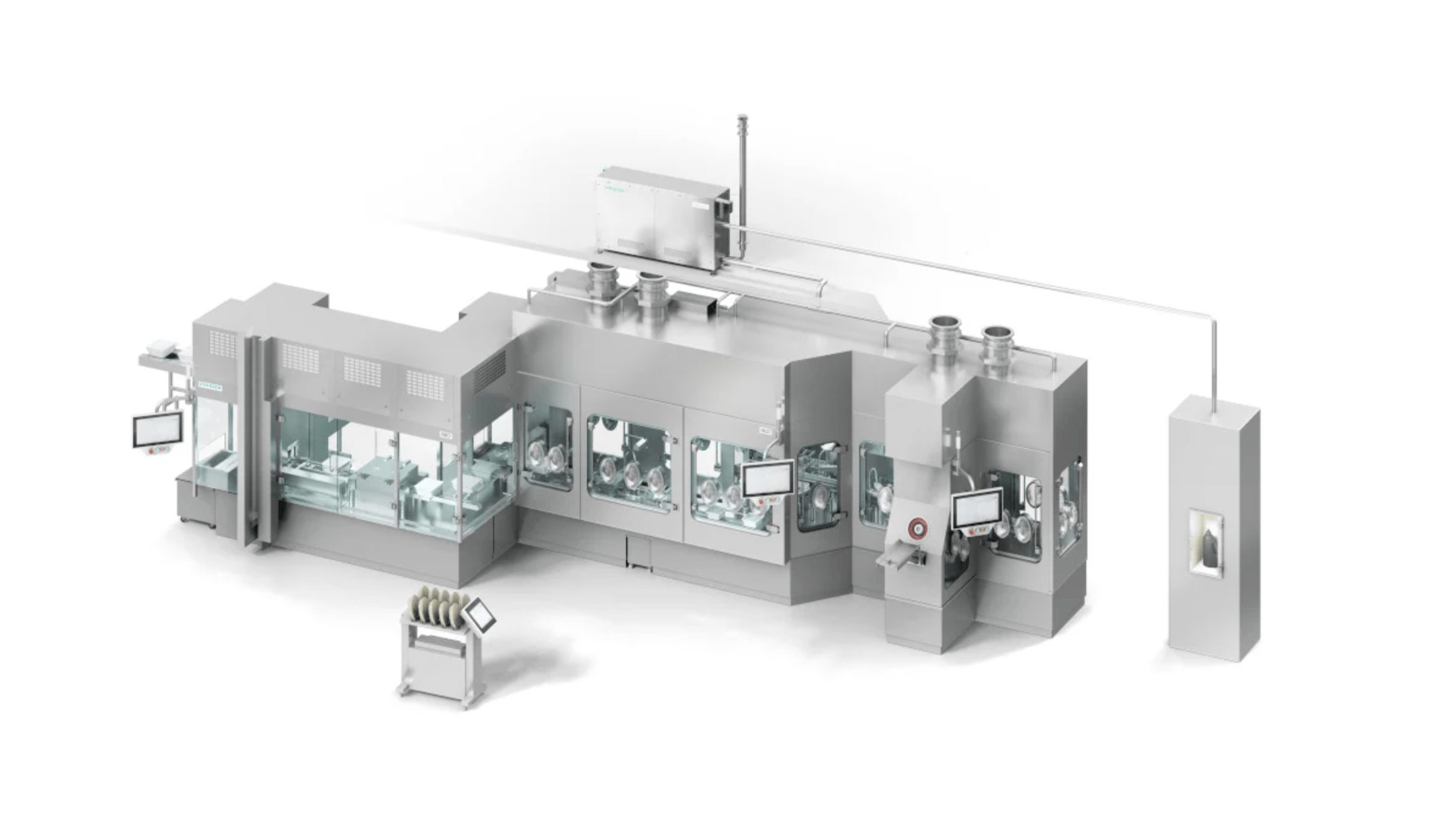
An innovative combination of proven technologies
The automatic bag and tub openers, well-known from many syringe lines, use no-touch transfer to ensure the aseptic transfer of the syringes into the filling area. The Pharma Handling Unit developed by Syntegon denests the syringes automatically without glass-to-glass contact and places the containers into the pitch adjustment station. Here, the syringes are moved into the machine pitch and then placed in the clips of the machine transport. “This unique clip system is the first key feature we adopted for syringe applications from the cartridge line,” Markus Burkert explains. “The special circulating transport system ensures smooth transportation and is therefore ideal for RTU syringes, as well as vials and cartridges.”

The empty syringes are weighed using 100 percent IPC, before they are transferred to the filling station. The subsequent filling and stoppering process is based on the proven FXS series for syringes: the filling needles are mounted on the Pharma Handling Unit and allow for flexible redosing if required. Thanks to IPC, the filling weight is controlled precisely.
“In addition to minimizing product loss, an important focus is on providing documented proof about the weighing of each container. This gives manufacturers the certainty that all containers have been filled correctly,” explains Klaus Ullherr, Senior Product Manager at Syntegon. After stopper insertion via vacuum or vent tube, the containers are returned by the circulating transport system and gently placed in the nests by the Pharma Handling Unit. “This way, we can offer our customers a seamless process for their pre-sterilized syringes from a single source,” says Klaus Ullherr.
This article was originally published by Syntegon.
Related News
-
Supplier News
Syntegon launches AIM9 high-speed inspection platform
-
Supplier News
Future-proof VFFS packaging from Syntegon
-
Supplier News
Syntegon opens new assembly hall in Remshalden to support future growth in its food business
-
Supplier News
Syntegon delivers strong growth and margin expansion in Q3 2025 with accelerating pharma momentum and profitability gains across all segments
-
Supplier News
Syntegon delivers strong growth and EBITDA improvements in first half of 2025





